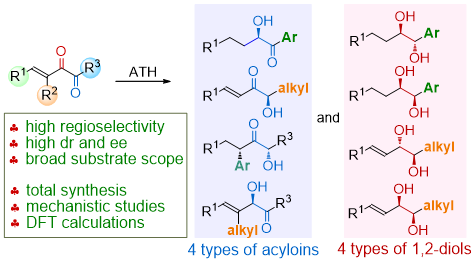
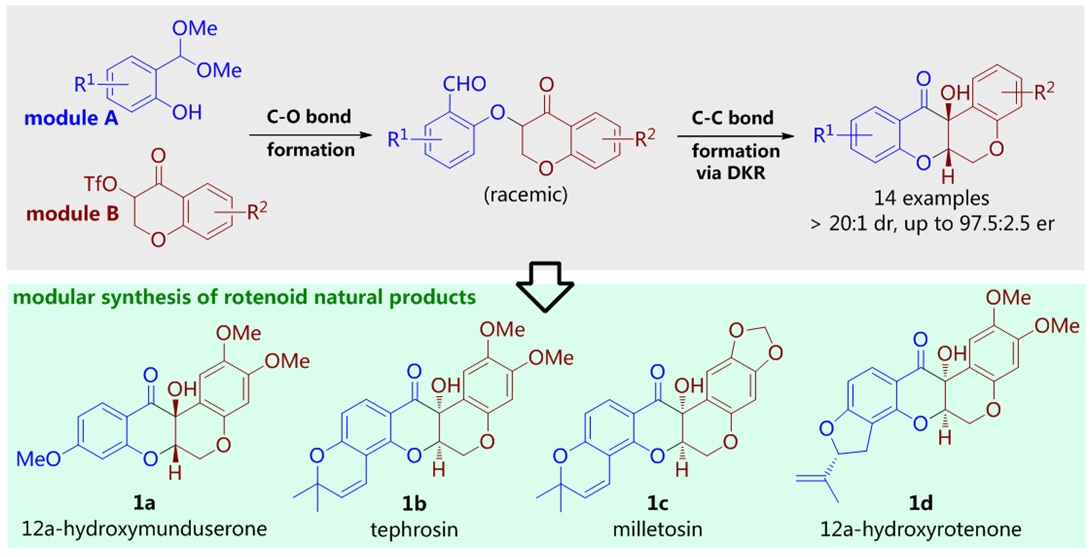
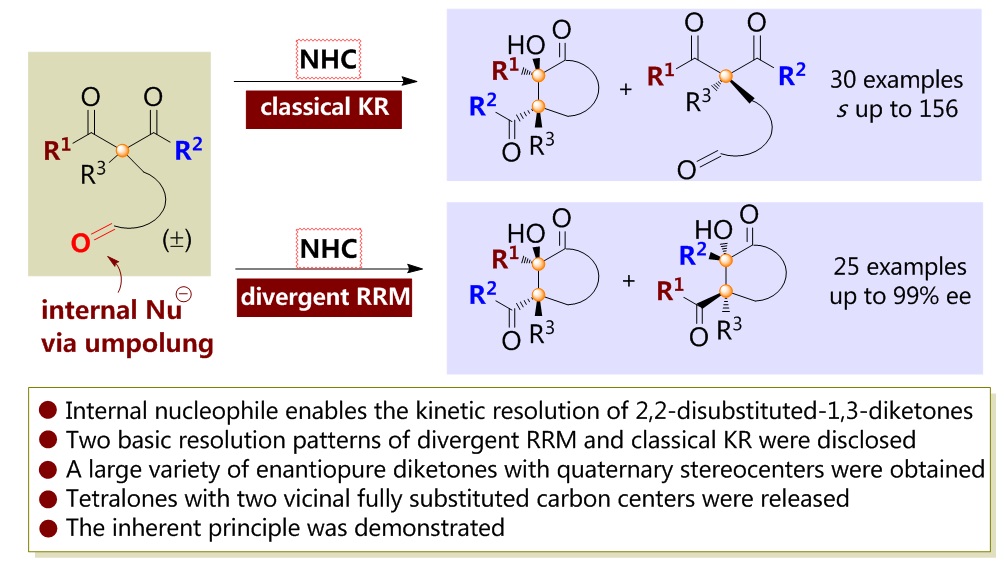
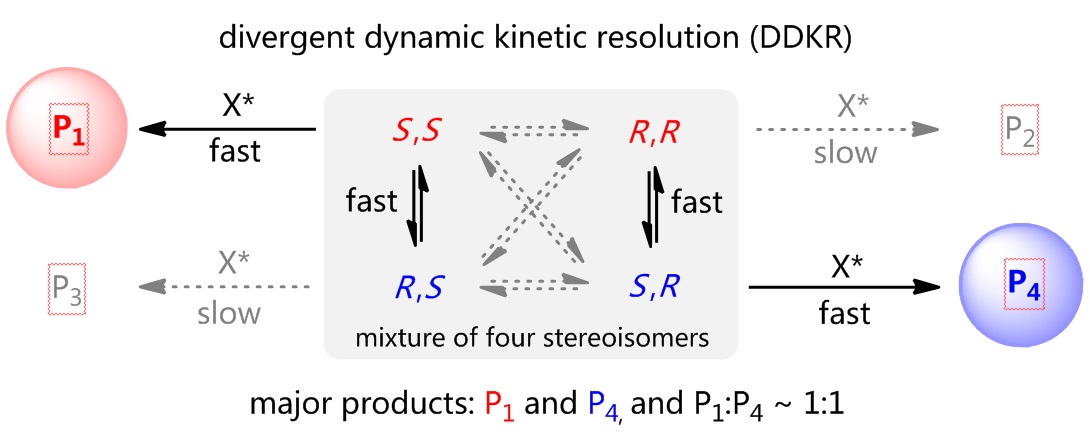
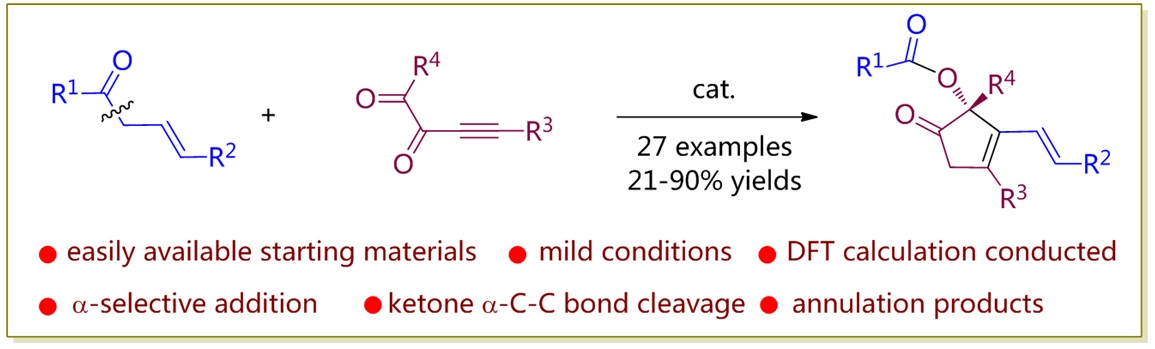
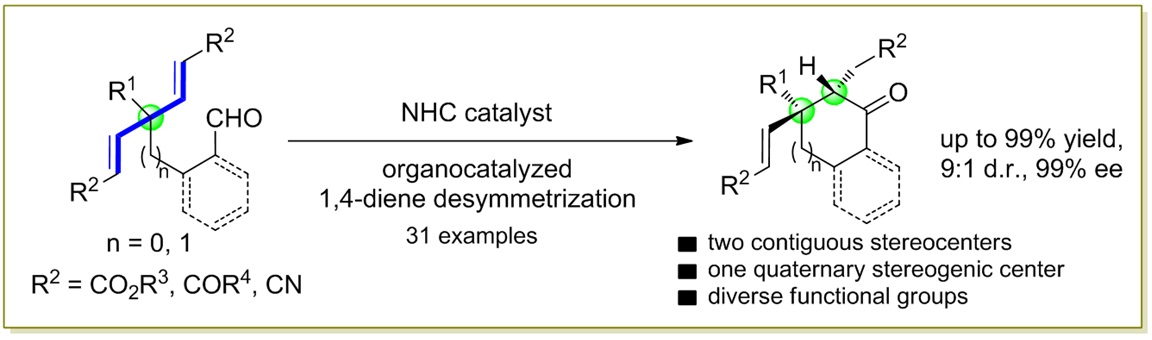
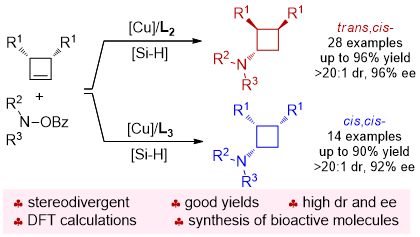
81. CuH-Catalyzed Asymmetric Desymmetrization of Cyclobutenes via Stereodivergent Hydroamination. Xiang Lei,§ Peng Luo,§ Shouang Lan, Wennan Dong, Chao Xu, Jinggong Liu, Shuang Yang, Qi Zhang,* Xinqiang Fang* ChemistryEurope. Accepted.
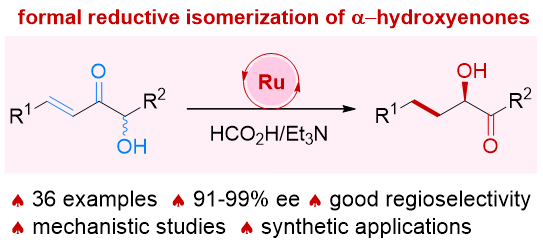
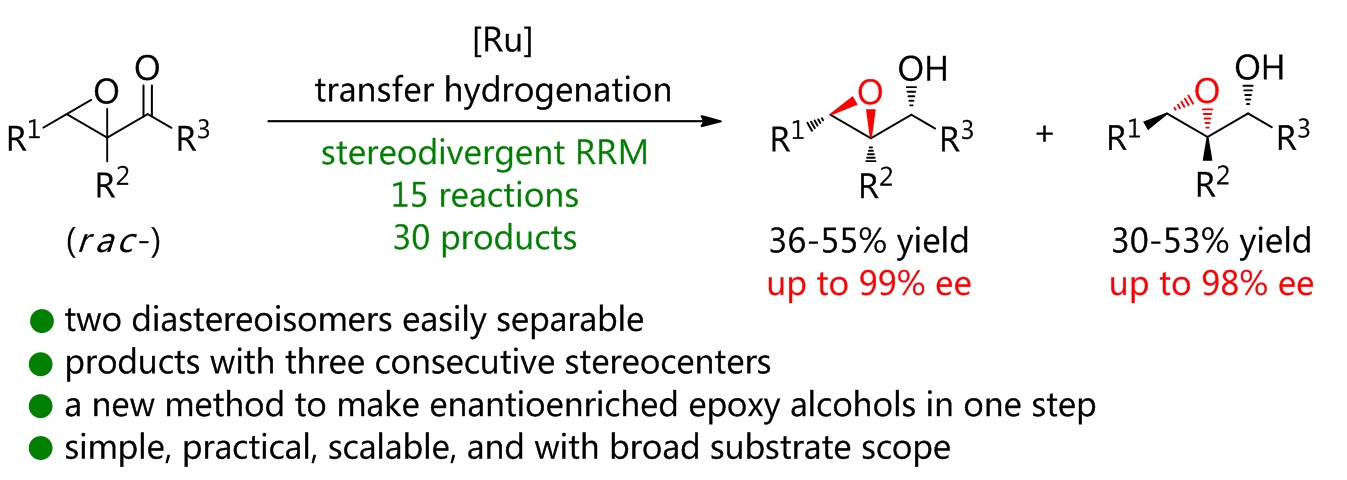
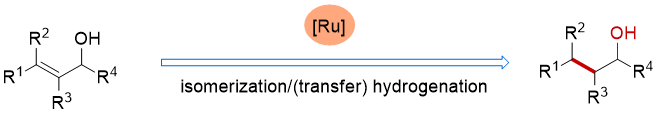
80. Ru-catalyzed isomerization/(transfer) hydrogenation of allylic alcohols. Shuang Yang,* Xinqiang Fang* Synthesis 2025, 57, 2423−2433. (Invited by Prof. Martin Oestreich)
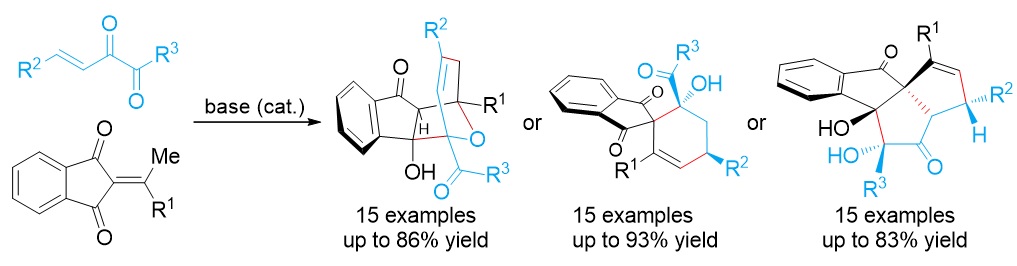
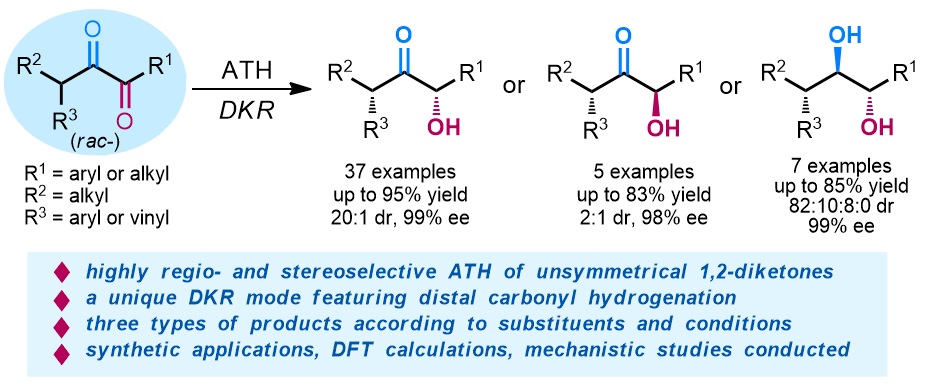
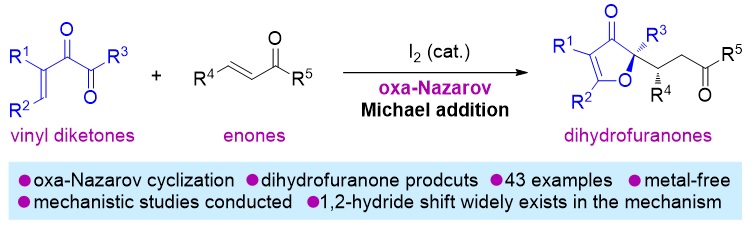
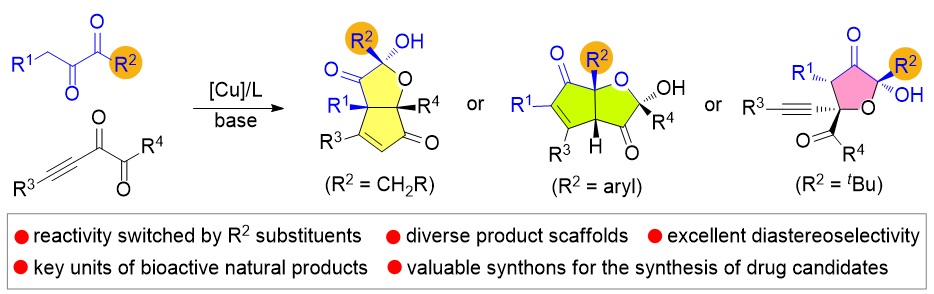
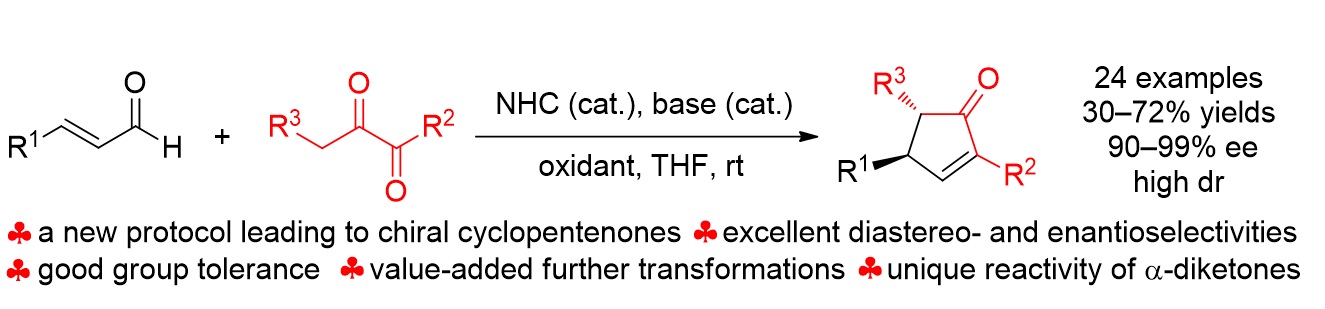
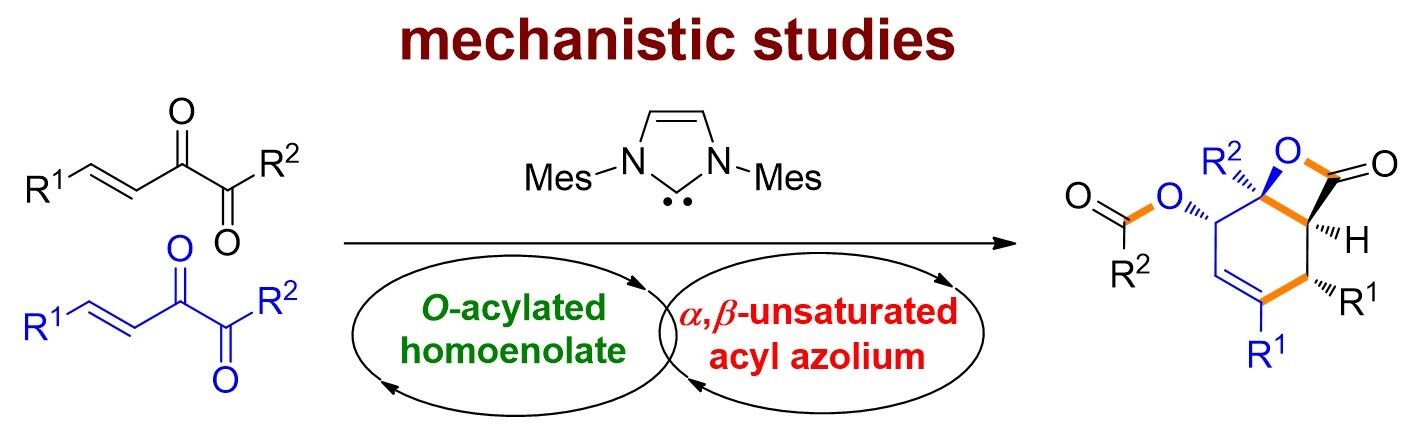
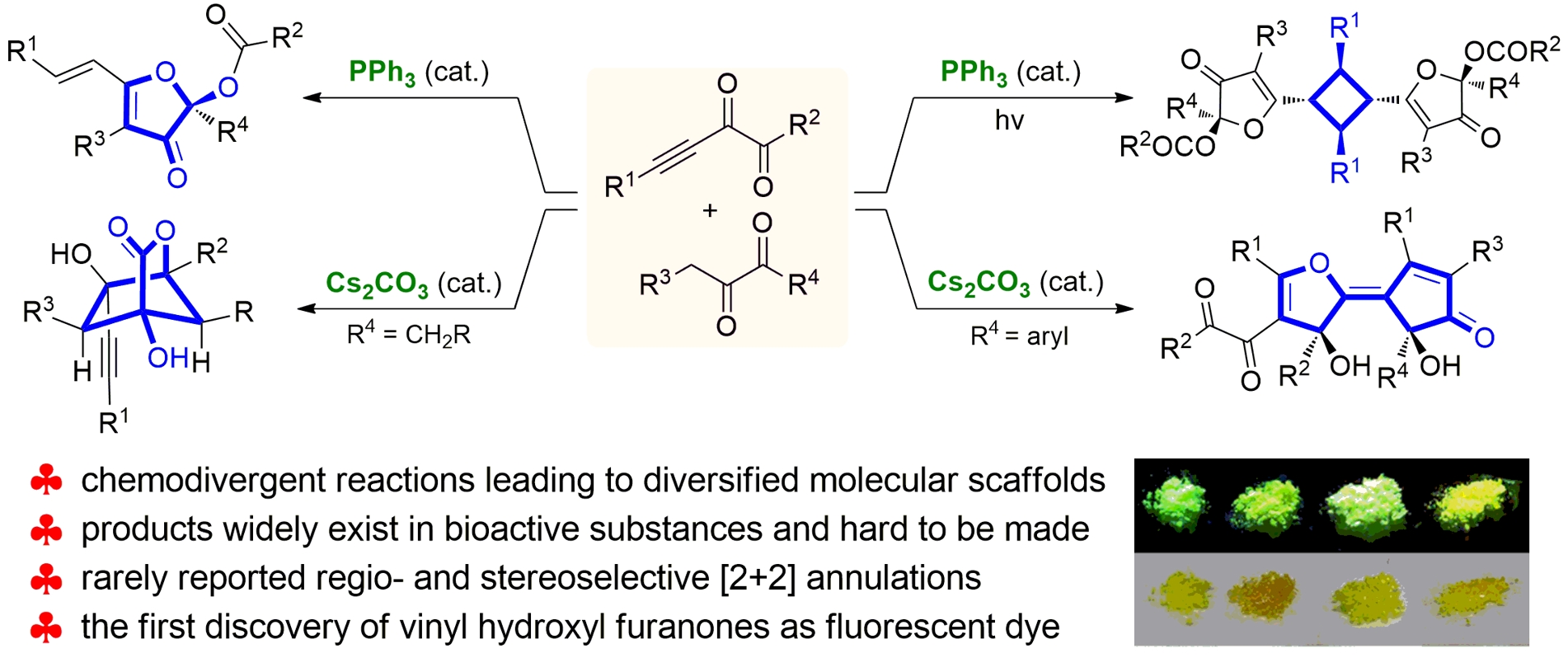
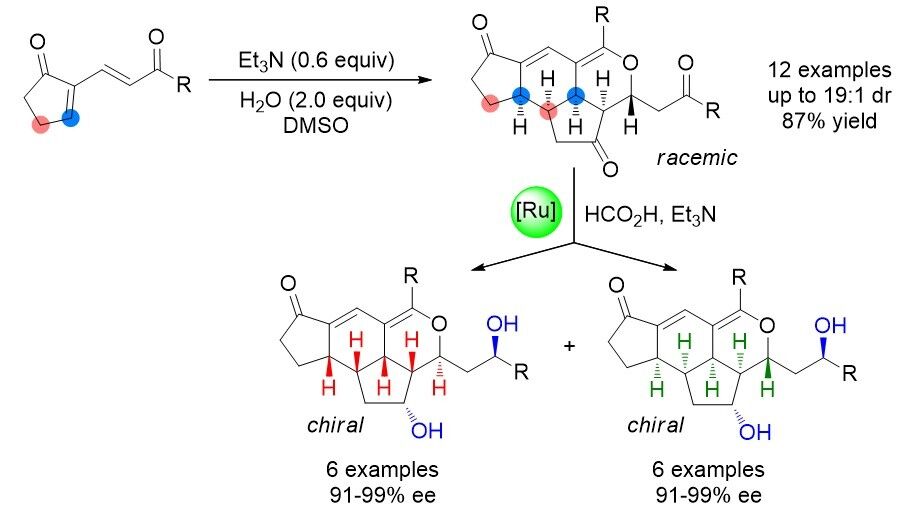
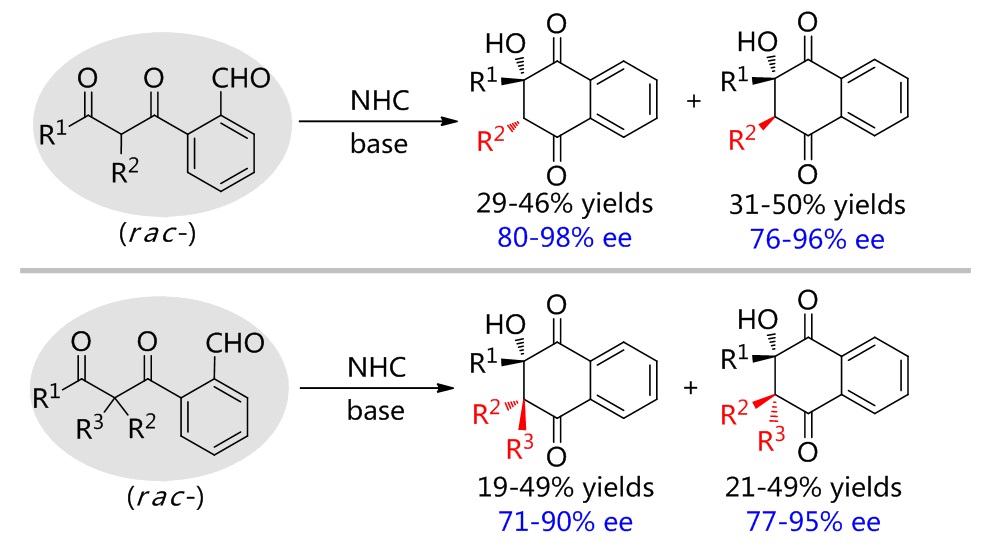
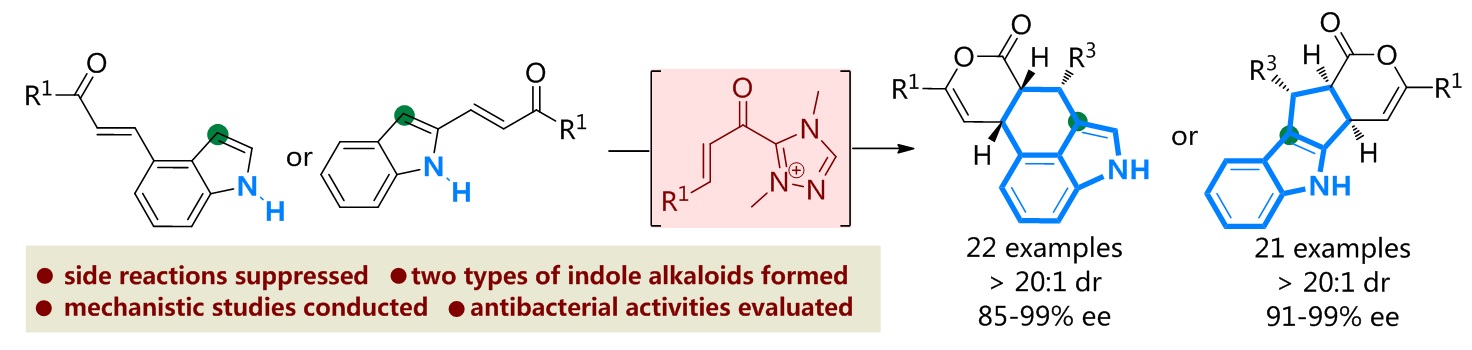
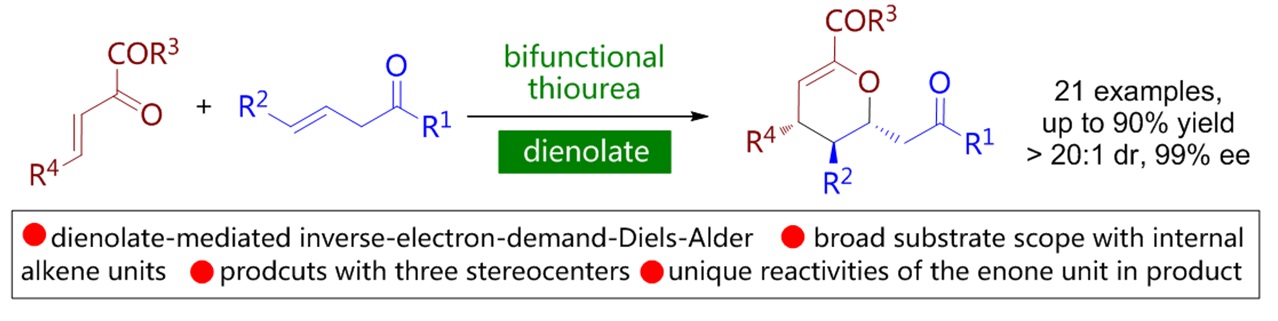
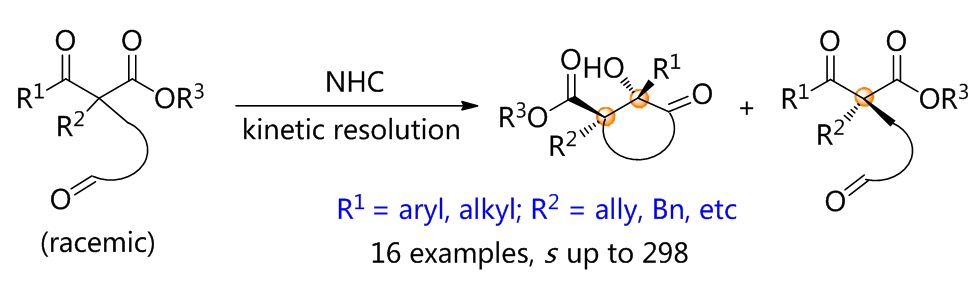
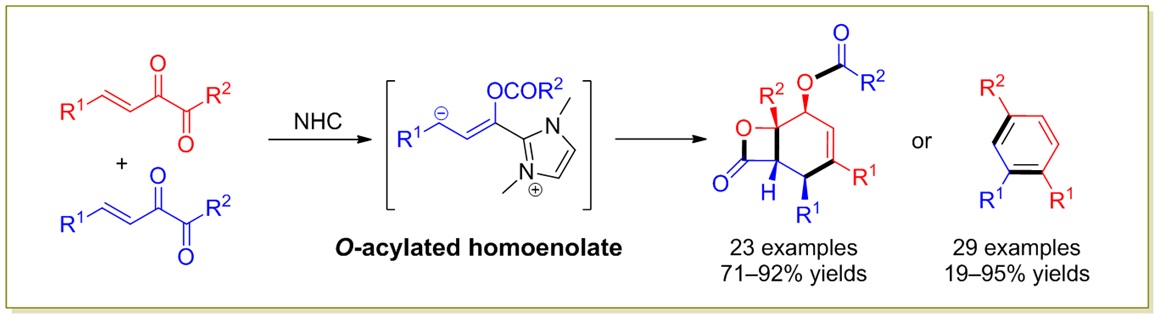
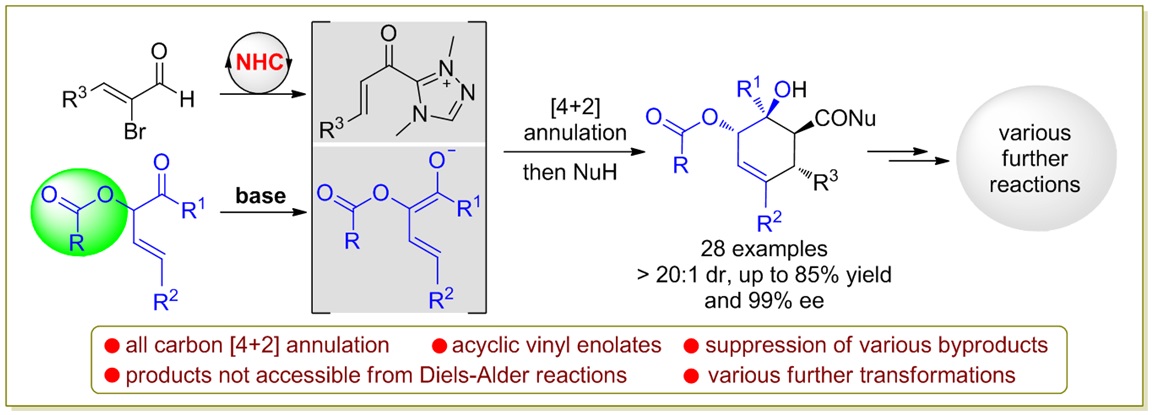
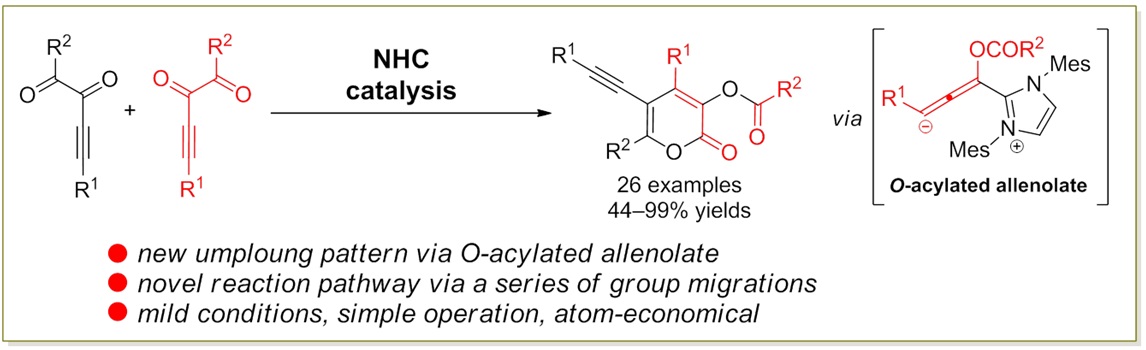
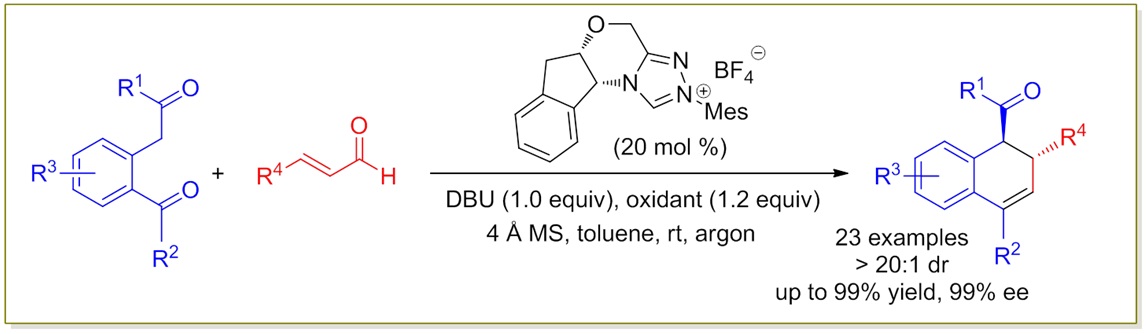
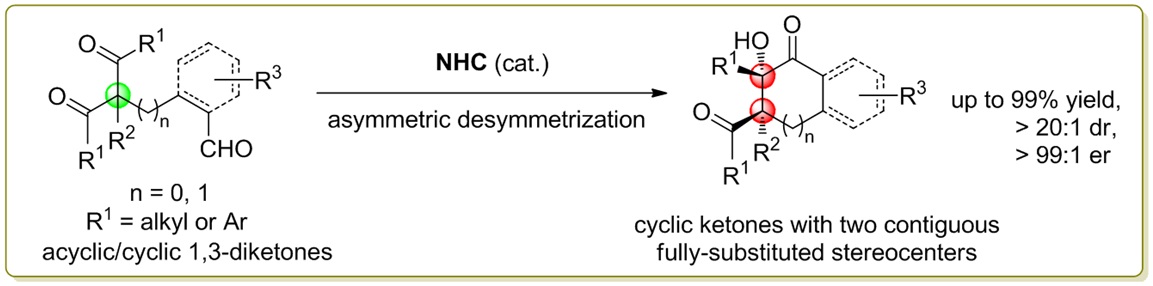
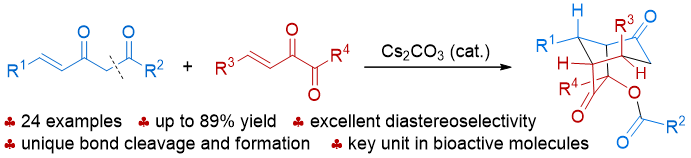
79. Catalytic Diastereoselective Synthesis of Bicyclo[3.2.1]octanediones. Liyuan Zhou,# Lili Zhao,# Caiyi Ren, Qinqin Cui, Shouang Lan, Chao Xu, Wennan Dong, Chunyun Jiang, Xiangwen Kong,* Benlong Luo,* Shuang Yang,* and Xinqiang Fang* Adv. Synth. Catal. 2025, 367, e202500324.
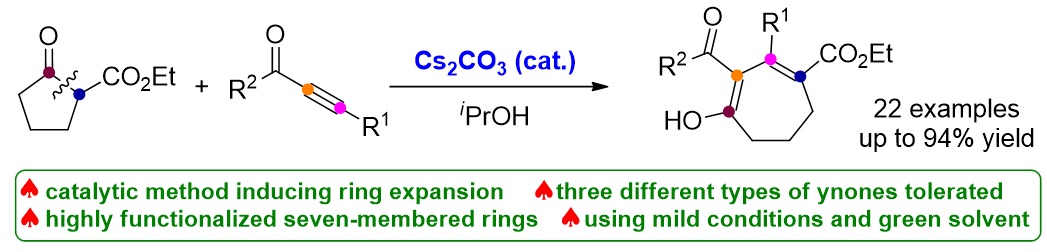
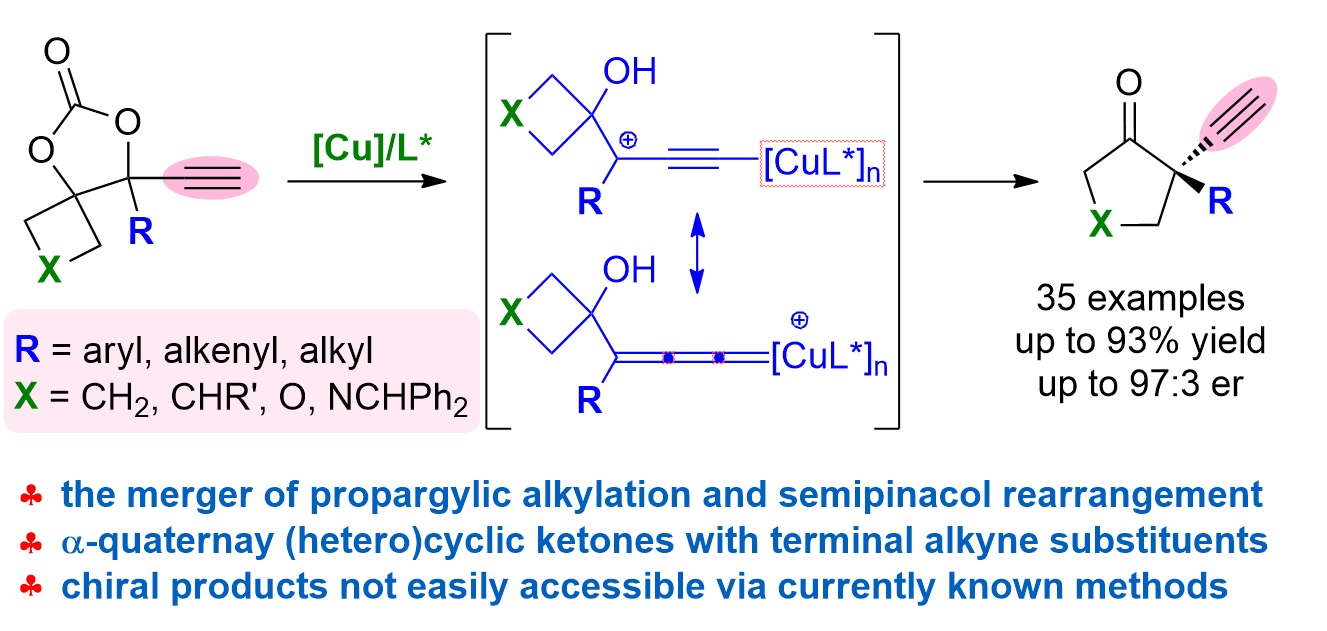
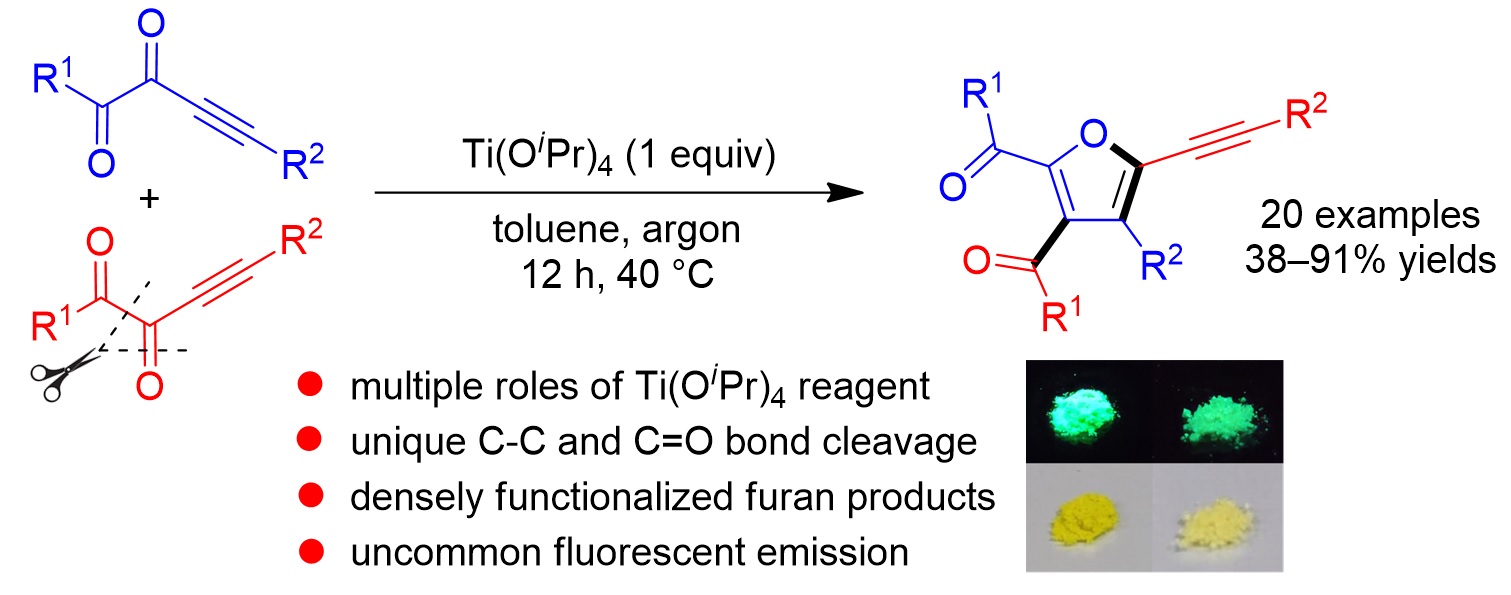
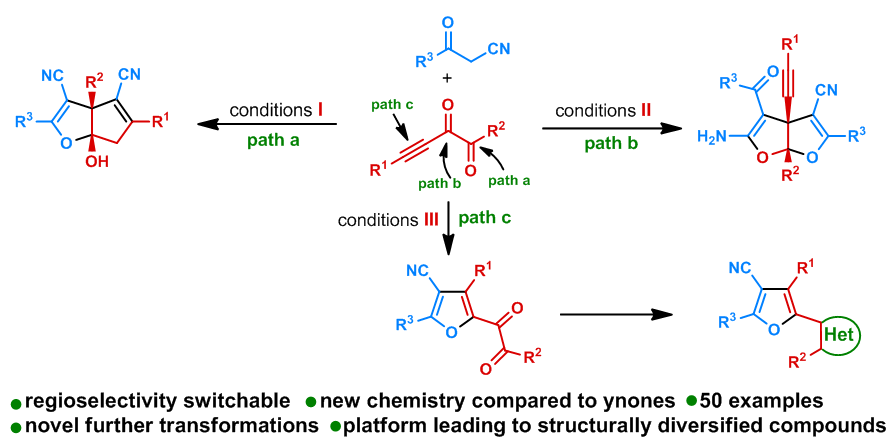
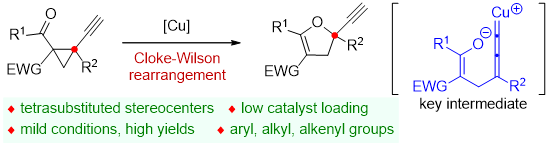
78. Copper-Catalyzed Cloke-Wilson Rearrangement for the Synthesis of Dihydrofurans Containing Tetrasubstituted Carbon Atoms. Qinqin Cui,§ Shouang Lan,§ Xiang Lei, Chao Xu, Chunyun Jiang, Lili Zhao, Liyuan Zhou, Jinggong Liu, Cheng-Zhi Gu,* Shuang Yang,* Xinqiang Fang* Org. Chem. Front. 2025, DOI: 10.1039/d5qo00401b.
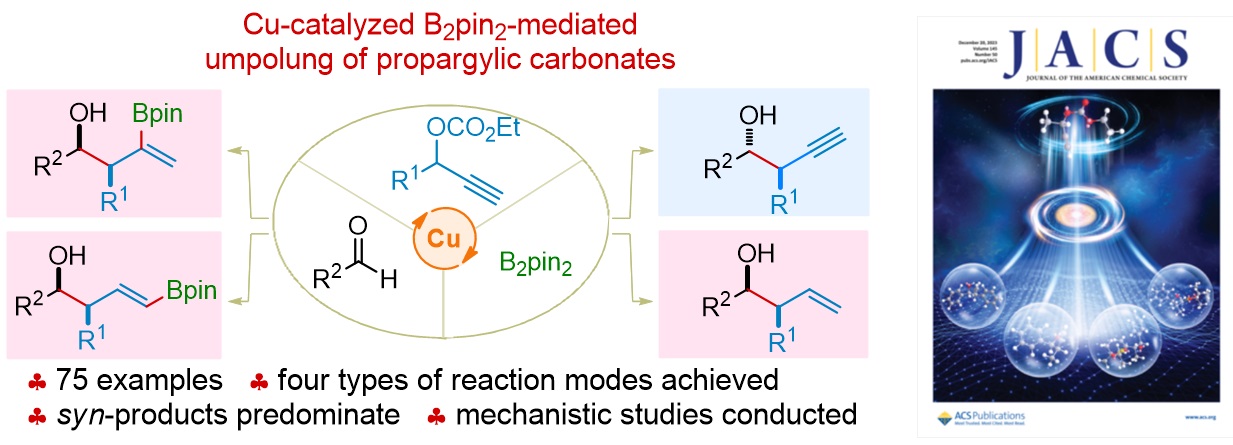
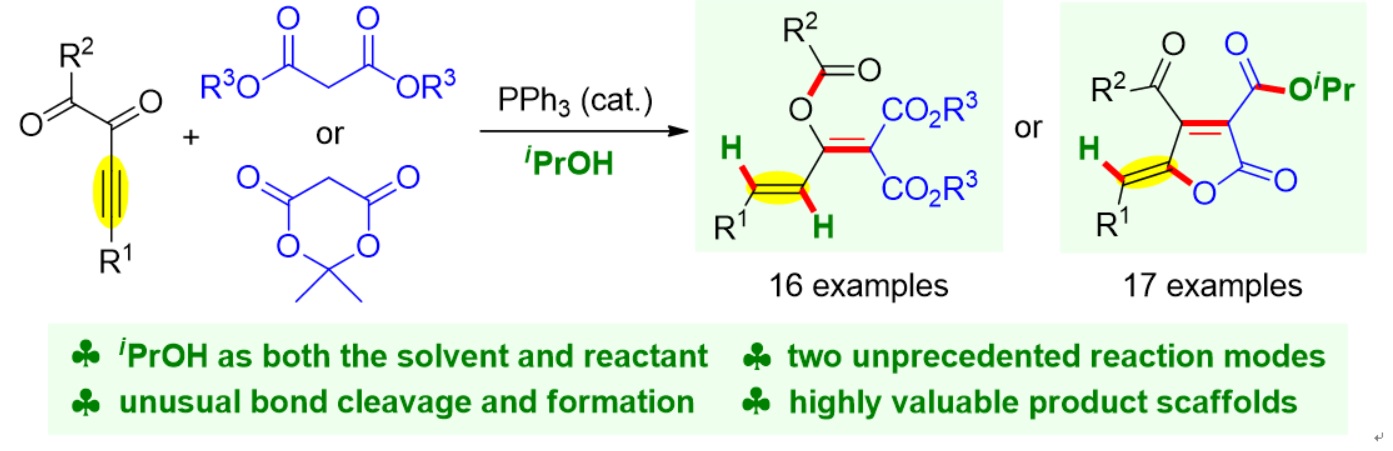
77. Copper-Catalyzed Dienylation of Aldehydes Using Propargylic Carbonates. Xiang Lei,§ Peng Luo,§ Wennan Dong,* Caiyi Ren, Qinqin Cui, Jinggong Liu, Xiang-Wen Kong, Shuang Yang,* Xinqiang Fang* Org. Lett. 2025, 27, 5355−5360.

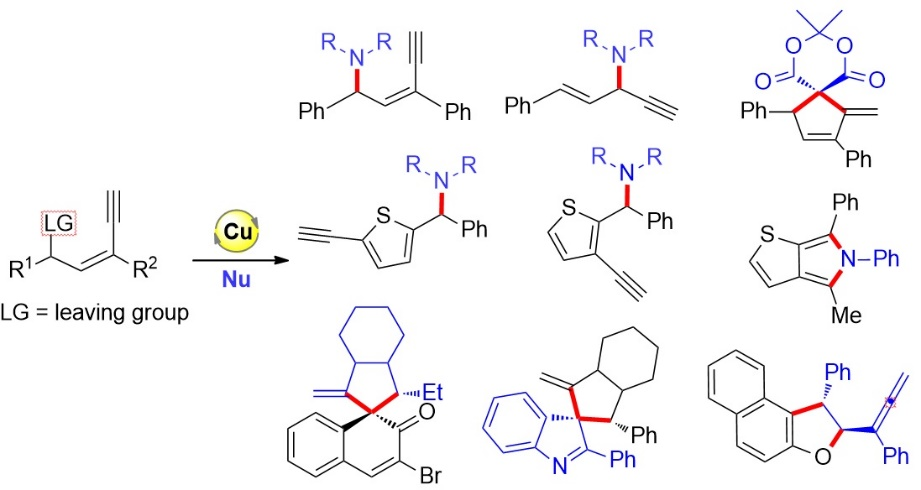
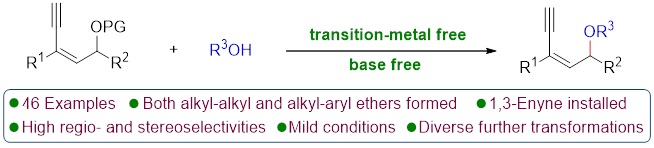
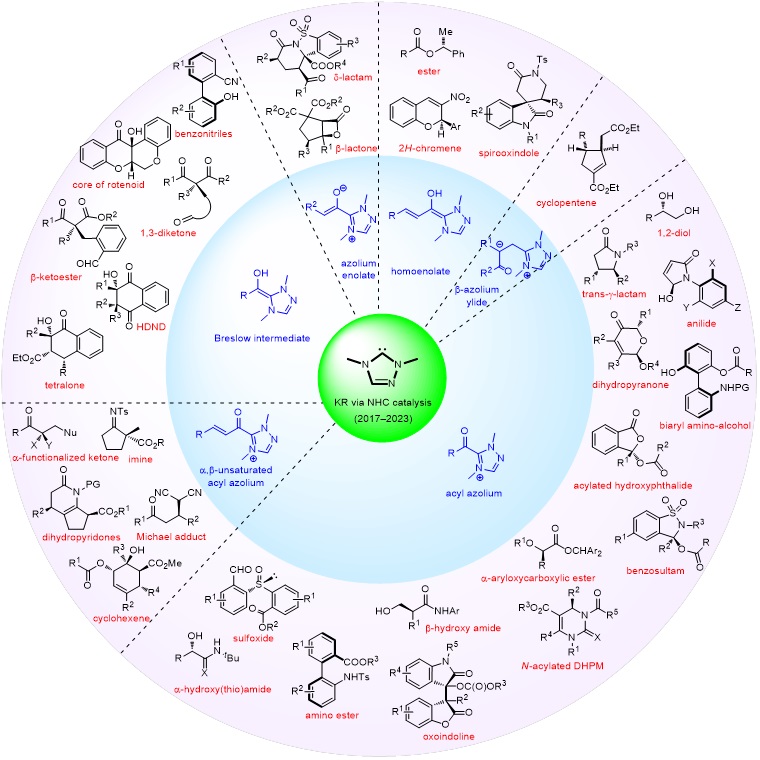
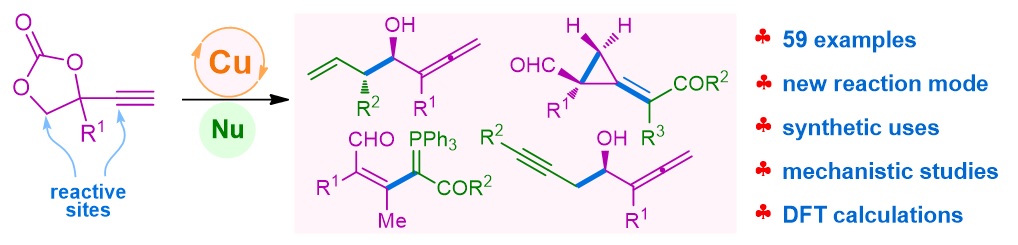
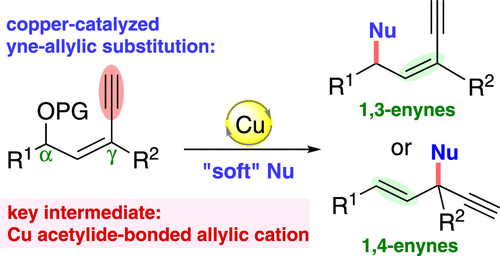
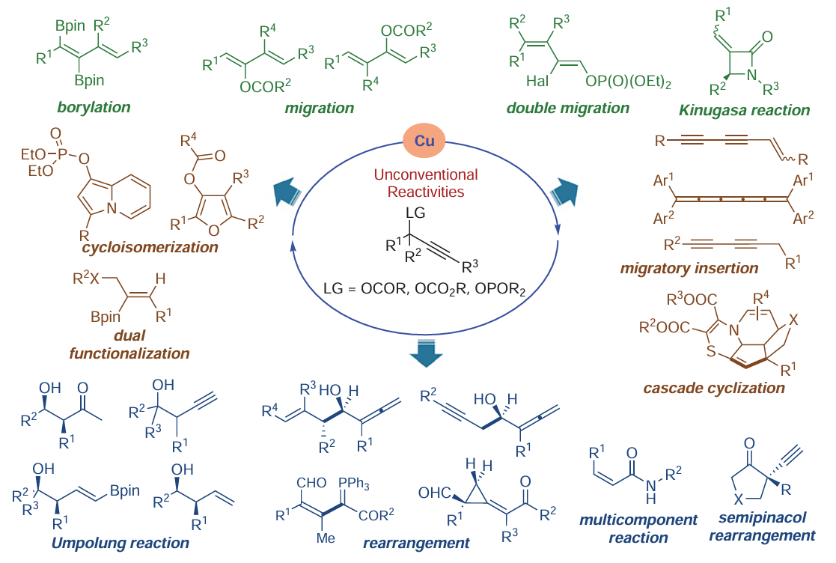
76. Copper-Catalyzed Unconventional Reactivities of Propargylic Esters. Shuang Yang,* Xinqiang Fang* Synlett 2024, 146, 33543−33560. (Invited by Prof. Debabrata Maiti)
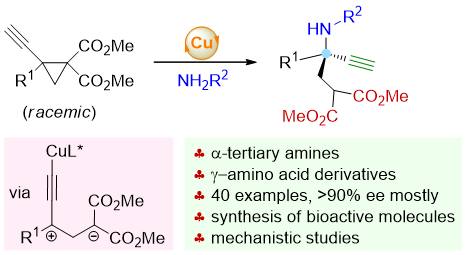
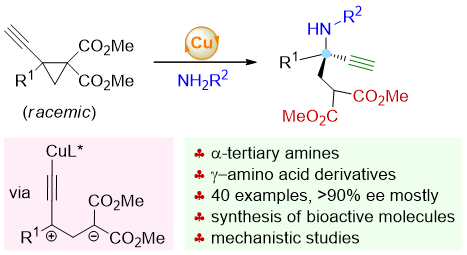
75. Copper-Catalyzed Asymmetric Nucleophilic Opening of 1,1,2,2-Tetrasubstituted Donor-Acceptor Cyclopropanes for the Synthesis of α-Tertiary Amines. Shouang Lan,§ Qinqin Cui,§ Defu Luo, Siyu Shi, Chengyang He, Shengyu Huang, Chao Xu, Lili Zhao, Jinggong Liu,* Cheng-Zhi Gu,* Shuang Yang, Xinqiang Fang* J. Am. Chem. Soc. 2025, 147, 1172–1185.